Assoc. Prof. in Biophysics at the Faculty of Medicine, University of Ljubljana 
Contact
Institute of biophysics, Faculty of Medicine, University of Ljubljana
Vrazov trg 2, 1000, Ljubljana, Slovenia
tel.: +386 1 5437600
e-mail: jure.derganc at mf.uni-lj.si
Google Scholar
You Tube channel: CellBiophysics
Short CV
- B.S. in physics, Faculty of Mathematics and Physics, University of Ljubljana (1997)
- Ph.D. in physics, Faculty of Mathematics and Physics, University of Ljubljana (2003)
- Training: Boston University (16 months); Institut Curie, Paris (3 months)
- Employment: teaching position at the Faculty of Medicine, University of Ljubljana (2003-present)
- Awards: Lavrič award for outstanding pedagogical work at the Faculty of Medicine, University of Ljubljana (2012)
Research
Biophysics of lipid membranes and cells
The function of living cells depends crucially on the biophysical properties of their membranes and cellular structures. To study cellular biophysics, we combine advanced theoretical modeling with state-of-the-art experimental methods such as optical tweezers, micropipette manipulation, confocal microscopy and microfluidics. The topics we study include bottom-up synthetic biology, membrane remodeling in cellular processes, interaction of pore-forming proteins and peptides with membranes and the role of cytoskeleton in cellular mechanics. The examples below show thin membrane nano-tubes pulled from a synthetic lipid vesicle using optical tweezers, and a cartoon showing how we use optical tweezers to probe cell elasticity.
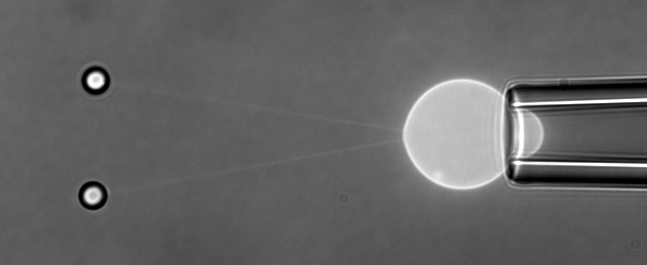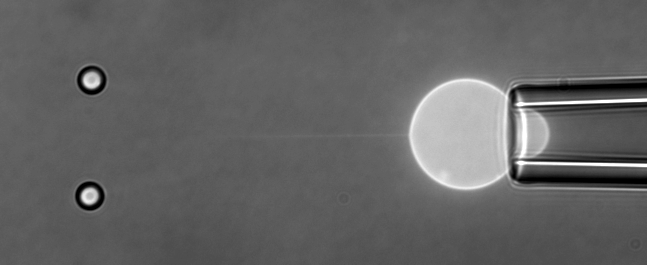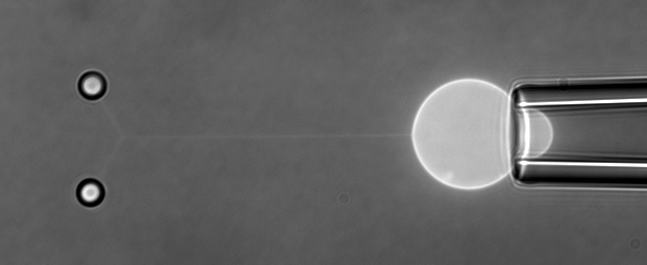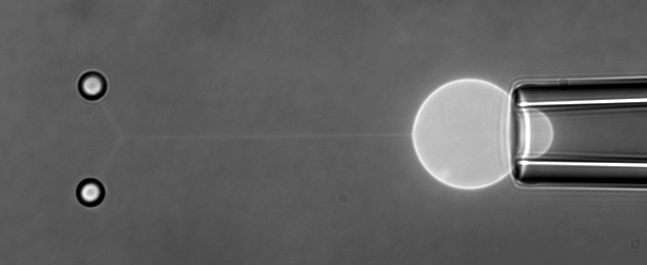
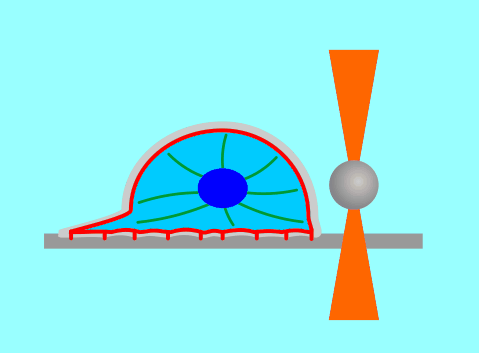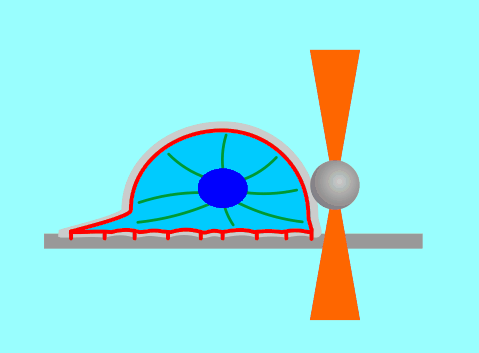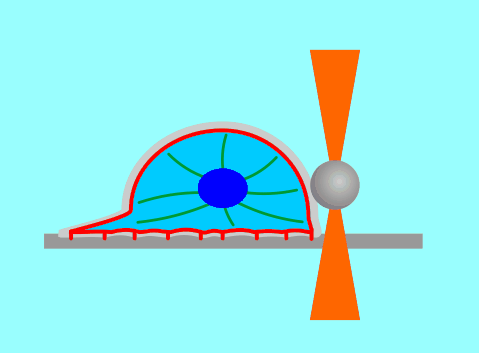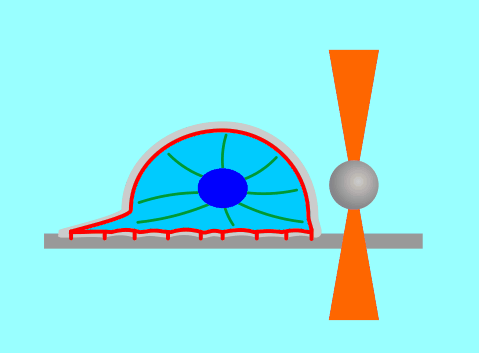
Current projects: out-of-equilibrium synthetic biology
A hallmark of living systems is their ability to thrive under non-equilibrium conditions. However, current experimental methods for manipulating and analyzing cells in controlled non-equilibrium conditions are limited. To address this, we are developing a high-throughput microfluidic system designed to create well-controlled chemical gradients that mimic the non-equilibrium conditions encountered in nature. The system will incorporate an advanced feedback control for flow, based on fast image acquisition. Once completed, the system will offer wide range of applications in the life sciences, such as:
- Assembly of a synthetic chloroplast. Many important cellular processes take place in membrane compartments with large area-to-volume ratios, such as the Golgi, the ER or the chloroplast. However, how to build such compartments in synthetic systems remains a mystery. One of our long-term goals is to develop tools for controlled shaping of membranes, e.g., for membrane origami, which would pave the way to a synthetic chloroplast that would be even more efficient than the natural one!
- Analysis of plant toxins. We have all heard of the Great Irish Famine of 1848 caused by potato blight (Phytophthora infestans). Much less known is that more than 150 years later, the molecular mechanisms of blight toxicity are still not understood! That is why we are working on it! Collaboration with the Department of Molecular Biology and Nanobiotechnology of the National Institute of Chemistry.
- Assessment of the response of human white blood cells to immunogenic stimuli. Immunology is complex. We need more tools to analyze the responses of white blood cells to external stimuli
Biomedical applications of microfluidics
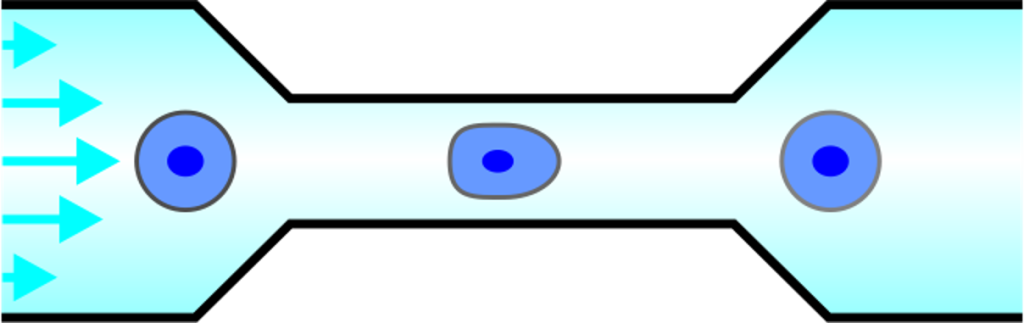
Microfluidic systems provide a versatile platform for biomedical experiments which cannot be performed in a standard test tube or Petri dish. The channels in such systems are typically narrower than a human hair! We focus on development of new microfluidic systems for high-throughput single-cell analysis. One example of our research is a flow-free microfluidic diffusion chamber, which allows for accurate analysis of floating cells during changes of their chemical environment.
The example below shows a schematic design of the diffusion chamber, the flow past the chamber, and a burst of a white blood cell in a chamber exposed to water.
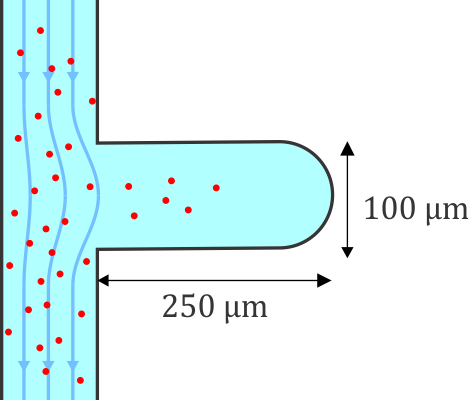
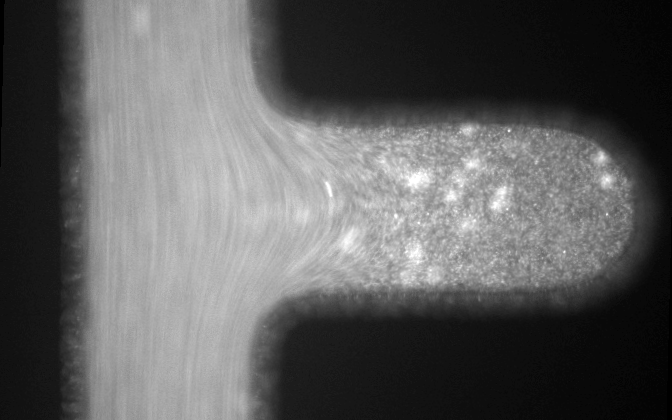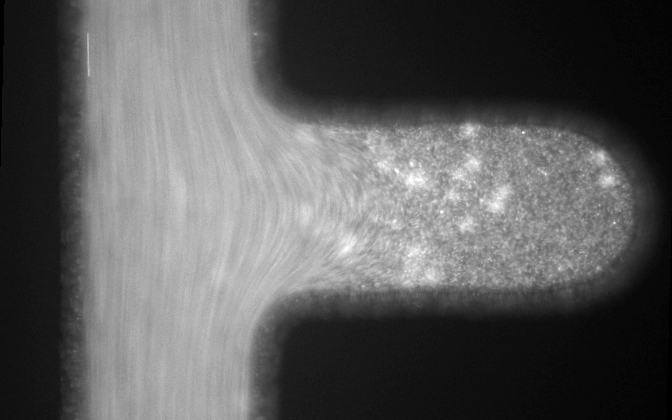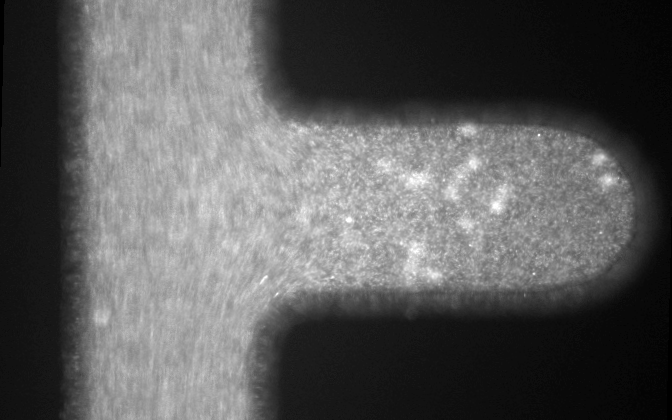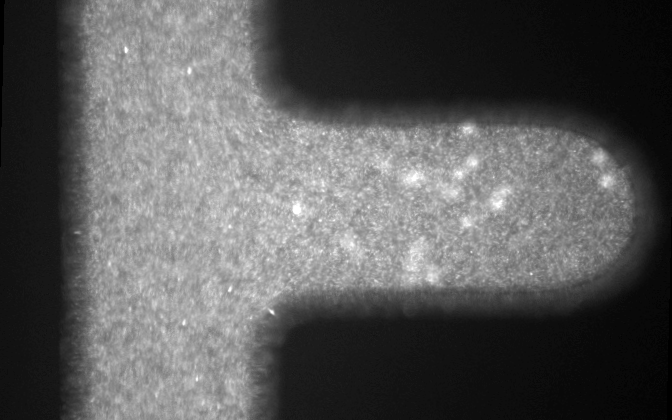
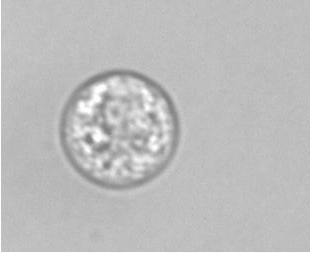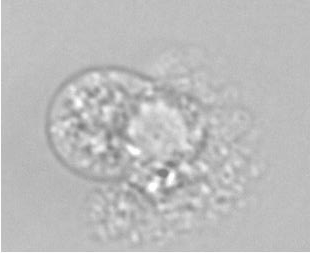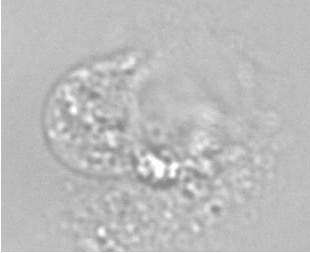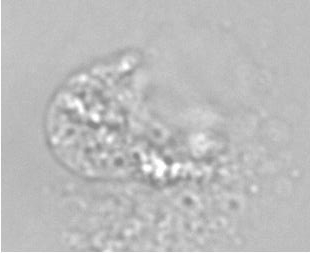
Current projects:
1. Deformability cytometry of lymphocytes for CAR-T therapy
2. Microfluidic mechanoporation for cell therapy (project J7-4493 supported by ARIS)
The example below shows high-throughput phenotyping of single model lymphocytes with Deformability Cytometry. In this method, the cells are pushed one-by-one at high speed through a narrow microchannel and deform due to high shear forces. We can analyze 500 cells per second and measure their size, deformability and morphology. Oh, and we are glad you asked – of course we are also developing advanced AI tools to analyze the cells!
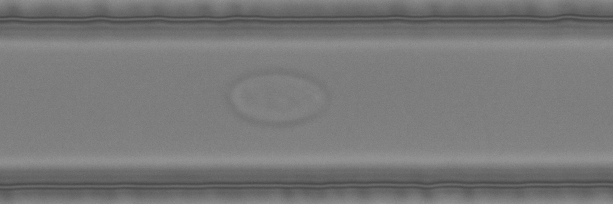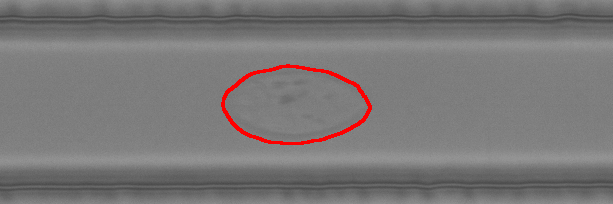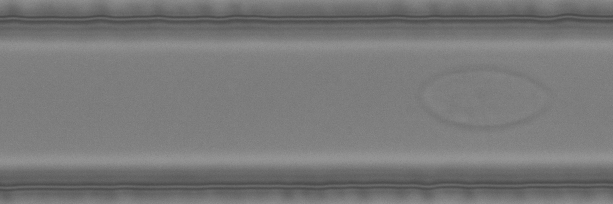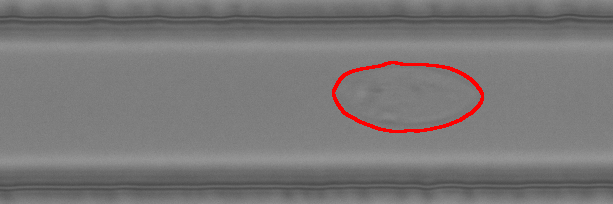
Selected publications
- Derganc, J., Zemljič-Jokhadar, Š., Majaron, B. and Kokot, G., 2023. Locally induced shockwaves for selective perforation of cargo loaded lipid vesicles with temporal and spatial control. RSC advances, 13(35), pp.24830-24834.
- Kuzman, D., Klančnik, U., Grum, E. and Derganc, J., 2023. Real-Time Assessment of the Size Changes of Individual Sub-Visible Protein Particles under Buffer Variations: A Microfluidic Study. Pharmaceuticals, 16(7), p.1002.
- Tuljak, M., Lajevec, D., Štanc R., Zemljič-Jokhadar, Š. and Derganc, J. 2022. Low-cost programable stroboscopic illumination with sub-microsecond pulses for high-throughput microfluidic applications. HardwareX, e00367.
- Pirc, Katja, et al., 2022. An oomycete NLP cytolysin forms transient small pores in lipid membranes. Science advances, eabj9406.
- Zemljič-Jokhadar, Š., Kokot, G., Pavlin, M. and Derganc, J., 2021. Adhesion and Stiffness of Detached Breast Cancer Cells In Vitro: Co-Treatment with Metformin and 2-Deoxy-d-glucose Induces Changes Related to Increased Metastatic Potential. Biology, 10(9), p.873.
- Gimenez-Andres, M., Emeršič, T., Antoine-Bally, S., D’Ambrosio, J.M., Antonny, B., Derganc, J. and Čopič, A., 2021. Exceptional stability of a perilipin on lipid droplets depends on its polar residues, suggesting multimeric assembly. Elife, 10, e61401.
- Jokhadar, S.Z., Iturri, J., Toca-Herrera, J.L. and Derganc, J., 2020. Cell Stiffness under Small and Large Deformations Measured by Optical Tweezers and Atomic Force Microscopy: Effects of Actin Disruptors CK-869 and Jasplakinolide. Journal of Physics D: Applied Physics, 54, 124001.
- Hartman, S.V., Božič, B. and Derganc, J., 2018. Migration of blood cells and phospholipid vesicles induced by concentration gradients in microcavities. New biotechnology, 47, pp.60-66.
- Mally, M., Božič, B., Hartman, S.V., Klančnik, U., Mur, M., Svetina, S. and Derganc, J., 2017. Controlled shaping of lipid vesicles in a microfluidic diffusion chamber. RSC advances, 7(58), pp.36506-36515.
- Marvin, T., Derganc, J. and Battelino, S., 2017. Adapting the Freiburg monosyllabic word test for Slovenian. Linguistica, 57(1), pp.197-210.
- Derganc, J. and Čopič, A., 2016. Membrane bending by protein crowding is affected by protein lateral confinement. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1858(6), pp.1152-1159.
- Osterman, N., Derganc, J. and Svenšek, D., 2016. Formation of vortices in long microcavities at low Reynolds number. Microfluidics and Nanofluidics, 20(2), p.33.
- Derganc, J., Antonny, B. and Čopič, A., 2013. Membrane bending: the power of protein imbalance. Trends in biochemical sciences, 38(11), pp.576-584.
- Vrhovec, S., Mally, M., Kavčič, B. and Derganc, J., 2011. A microfluidic diffusion chamber for reversible environmental changes around flaccid lipid vesicles. Lab on a Chip, 11(24), pp.4200-4206.
- Derganc, J., 2007. Curvature-driven lateral segregation of membrane constituents in Golgi cisternae. Physical Biology, 4(4), p.317.
- Derganc, J., Mironov, A.A. and Svetina, S., 2006. Physical factors that affect the number and size of Golgi cisternae. Traffic, 7(1), pp.85-96.
Supported by

